D. E. Russell1, A. Gunn2, S. Kutz3
1CircumArctic Rangifer Monitoring and Assessment (CARMA) Network, Yukon College, Whitehorse, YT, Canada
2CARMA, Salt Spring Island, BC, Canada
3Department of Ecosystem and Public Health, Faculty of Veterinary Medicine, University of Calgary, Calgary, AB, Canada
Highlights
- The abundance of migratory tundra caribou and wild reindeer has continued to drop since declines were detected in the mid-1990s.
- Of the 22 herds monitored, only two herds are at historic peak numbers and have not declined.
- Recent analyses link caribou productivity, particularly declining calf and adult survival, to changing climate conditions.
- Current low numbers of caribou and wild reindeer have imposed hardships for northern communities.
The abundance of migratory herds of caribou (North America and Greenland) and wild reindeer (Russia and Norway) in circum-arctic tundra regions (Fig. 1) has declined 56% (4.7 million to 2.1 million) over the last two decades. Five herds in particular, in the Alaska-Canada region, have declined more than 90% and show no sign of recovery (Fig. 2). It is normal for herd numbers to vary over decades (Fauchald et al., 2017), but currently some herds have all-time record low populations since reliable record keeping began. The extent and duration of the declines are a threat to the food security and culture of indigenous people who have depended on the herds. Caribou and wild reindeer are a key species in the arctic food web contributing to nutrient cycling between terrestrial and aquatic systems and the abundance of predators and scavengers.

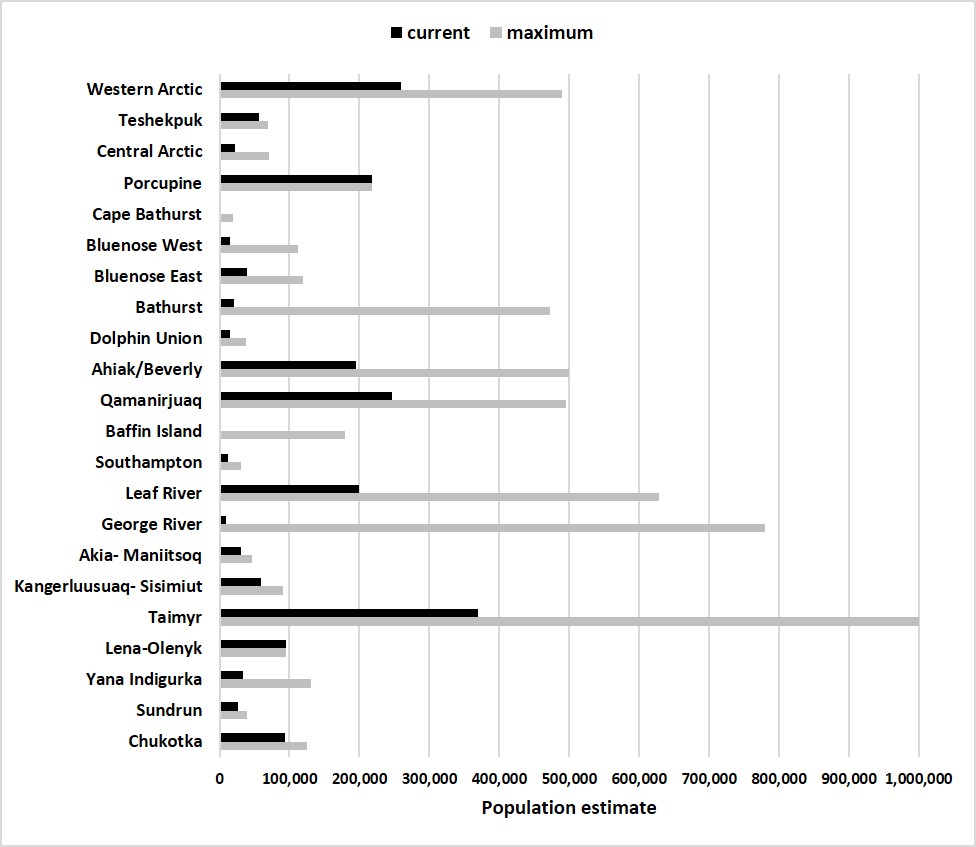
The status of the Arctic tundra caribou and wild reindeer herds (Fig. 2) is monitored by the CircumArctic Rangifer Monitoring and Assessment (CARMA) Network. CARMA relies on collaboration among scientists, wildlife management agencies, management boards, and Native organizations to share their data and information on caribou and wild reindeer herds. The CARMA Network maintains a relatively new, publicly accessible database of herd observations and descriptions (https://carma.caff.is/) collected via data sharing efforts, although accessible data archiving is still in its infancy. The CARMA database includes data for 23 herds (geographic sub-populations) with an average frequency of estimated herd size occurring every 5 years (0.47 Standard Error; range 2-10 years; Fig. 1).
Two aerial survey methods are used to estimate herd abundance. The first uses total counts based on photography of mid-summer aggregations as the caribou and wild reindeer gather to reduce their exposure to insect harassment (e.g., Klimstra, 2018, unpubl. comm.). The second method uses a sample count based on photography of calving grounds where the cows of a herd are known to come together for calving. Calving ground sample counts are then extrapolated, using adult sex ratios, to estimate herd size (e.g., Adamczewski et al., 2017).
Regional variation in the extent and timing of the caribou declines is high. For example, of the five herds tracked in Alaska, three herds peaked between 2003 and 2010 before precipitously declining 53% by 2017, while during the same period, two Alaskan herds began to recover. Notably, one of the Alaska herds, the Porcupine (shared with Canada), is the only herd in the state to recently increase in population size (44% between 2001 and 2016).
Across the Canadian Arctic mainland, declines in nine herds have become severe enough that barren-ground caribou became nationally recognized as “Threatened” (as defined by COSEWIC) in 2016 (COSEWIC, 2016) and two herds of Eastern Migratory Caribou are now considered “Endangered” (COSEWIC, 2017). In Russia, where there is a high diversity of wild reindeer sub-species, recent declines are especially apparent for island, forest, and mountain reindeer. Of 19 herds assessed, 18 are rare, decreasing, or “Threatened” (I. Mizin, pers. comm., 2018).
In western Greenland, caribou inhabiting mountain (alpine) tundra, recently declined and harvesting is consunpuidered a leading factor (Cuyler et al., 2011). In south-central Norway, where mountain reindeer also live in alpine tundra, there are an estimated 6,000 wild reindeer that have maintained relatively stable populations since 2002 (Strand et al., 2012).
Causes of the caribou and wild reindeer population declines are complex and are related to a combination of factors that include forage availability, macro (worms and ectoparasites) and micro (viruses, bacteria, protozoa) parasites, predation (including hunting), and climate change (an overarching factor). To examine the influence of climate, a spatial climate dataset (Russell et al., 2013) was created for annual and seasonal ranges of circumpolar caribou and wild reindeer populations. In comparing herd vital rates in North American herds, climate indicators accounted for 54% of the variability in vital rates. This study also revealed “carry-over” effects reflecting the adaptability of caribou to buffer adverse weather. For example, a series of years with adverse conditions such as drought resulted in reduced pregnancy and calf survival.
Caribou and wild reindeer are adapted to the long Arctic winters, which is likely why herd vital rates did not negatively correlate with winter snow variables (snow depth on 31 March). The strongest and most consistent climate trends were the extent of October warming growing degree days and also increasing plant growing degree days in June and July temperatures. While these trends, and trends for earlier snow loss, are often beneficial to caribou, subsequent warmer summers also have adverse effects through increased drought, flies and parasites, and perhaps heat stress leading to increased susceptibility to pathogens and other stressors.
Trends observed between individual herd and climate variables are in good agreement with trends observed in regional climate observations. For example, Bhatt et al. (2017) reported summer warming trends for the North American Arctic that align with observations of Bathurst (Canada) caribou adult cow mortality. In this example, correlations emerged between warmer summer temperatures and reduced July rainfall, which increased the drought index. The 2-year running average of mean monthly July temperature and cumulative freezing rain from September to December explained 64% of the variation in adult cow survival which, in turn, is a determining factor in herd size (Fig. 3). The exact mechanism for the lower survival is uncertain but may be linked at least in part to heat stress and the higher temperatures decreasing forage quality and reducing forage intake as digestive efficiency declines, while freezing rain reduces forage intake and increases movement rates.

Warmer summer temperatures have also been linked to increased abundance and range expansion of Arctic pathogens (Kutz et al., 2013; Altizer et al., 2013; Kafle et al., 2018). Increasingly, we are understanding the role of these endemic pathogens in limiting wild ungulate populations (e.g., Tryland and Kutz, 2018); however, we also need to be aware of unanticipated effects of climate changes on infectious disease in wild populations. For example, spring and summer warming has the potential to trigger unexpected die-offs. This phenomenon was observed in an ecologically similar species when, in June 2015, an outbreak of Pasteurella in the gregariously calving saiga antelope (Saiga tatarica) killed more than 200,000 antelopes (Kock et al., 2018).
Disease outbreaks in muskoxen in the Canadian Arctic (Kutz et al., 2015 ) were only recognized when local ecological knowledge was collected (Tomaselli et al., 2018). Subsequent surveys demonstrate this same bacteria is also circulating across all North American caribou herds tested and that it can cause caribou deaths (K. J. Bondo et al., unpubl. report: “Health status of live-captured boreal caribou in Northeastern British Columbia during year of unusually high mortality”; S. J. Kutz et al., unpubl. data). While recent research ties climate variables to caribou and wild reindeer vital rates, the cascading and interacting effects of climate, nutrition, infectious and non-infectious disease, predation, and disturbance on caribou and wild reindeer require in depth and multi-disciplinary investigation to better understand their effects on population dynamics and future population trends.
Regional differences in climate and other landscape changes such as roads and industrial developments mean that herds are exposed to different cumulative effects and their consequent vulnerability varies among herds. With spatial climate trends, and monitoring herds, including systematic collection of local ecological knowledge, we are increasingly better placed to assess herd vulnerability to promote adaptive capacity, which in turn will increase landscape resilience and support the adaptability of the arctic people.
References
Adamczewski, J., J. Boulanger, B. Croft, T. Davison, H. Sayine-Crawford, and B. Tracz, 2017: A comparison of calving and post-calving photo-surveys for the Bluenose-East herd of barren-ground caribou in northern Canada in 2010. Can. Wildlife Biol. Manag., 6, 4-30.
Altizer, S., R. S. Ostfeld, P. T. Johnson, S. Kutz, and C. D. Harvell, 2013: Climate change and infectious diseases: From evidence to a predictive framework. Science, 341(6145), 514-519.
Bhatt, U. S., et al., 2017: Changing seasonality of panarctic tundra vegetation in relationship to climatic variables. Environ. Res. Lett., 12, 055003, doi: 10.1088/1748-9326/aa6b0b.
COSEWIC, 2016: COSEWIC assessment and status report on the Caribou Rangifer tarandus, Barren-ground population in Canada. Committee on the Status of Endangered Wildlife in Canada (Ottawa), 136 pp.
COSEWIC, 2017: COSEWIC assessment and status report on the Caribou Rangifer tarandus, Eastern Migratory population and Torngat Mountains population, in Canada. Committee on the Status of Endangered Wildlife in Canada (Ottawa), 85 pp.
Cuyler, C., M. Rosing, H. Mølgaard, R. Heinrich, and K. Raundrup, 2011: Status of two West Greenland caribou populations 2010; 1) Kangerlussuaq-Sisimiut, 2) Akia-Maniitsoq, Part I. Pinngortitaleriffik – Greenland Institute of Natural Resources. Technical Report No. 78, 162 pp.
Fauchald, P., T. Park, H. Tommervik, R. Myneni, and V. H. Hausner, 2017: Arctic greening from warming promotes declines in caribou populations. Sci. Adv., 3, e1601365, doi: 10.1126/sciadv.1601365.
Kafle, P., S. J. Peacock, S. Grond, K. Orsel, and S. Kutz, 2018: Temperature-dependent development and freezing survival of protostrongylid nematodes of Arctic ungulates: Implications for transmission. Parasites Vectors, 11, 400, doi: 10.1186/s13071-018-2946-x.
Klimstra, R, 2018: Summary of Teshekpuk Caribou Herd photocensus conducted July 14, 2017. Unpubl. memo, Alaska Department of Fish and Game, Division of Wildlife Conservation Northwest, Fairbanks, Alaska.
Kock, R. A., M. Orynbayev, S. Robinson, S. Zuther, and N. Singh, 2018: Saigas on the brink: Multidisciplinary analysis of the factors influencing mass mortality events. Sci. Advances, 4, eaao2314.
Kutz, S. J., et al., 2013: Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Global Change Biol., 19(11), 3254-3262.
Kutz, S. J., et al., 2015: Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Can. Vet. J., 56(6), 560-563.
Russell, D. E., P. H. Whitfield, J. Cai, A. Gunn, R.G. White, and K. Poole, 2013: CARMA’s MERRA-based caribou climate database. Rangifer, 33(Special Issue 21), 145-152.
Strand, O., E. B. Nilsen, E. J. Solberg, and J. C. D. Linnell, 2012: Can management regulate the population size of wild reindeer (Rangifer tarandus) through harvest? Can. J. Zool., 90, 163-171.
Tomaselli, M, S. Kutz, C. Gerlach, and S. Checkley, 2018: Local knowledge to enhance wildlife population health surveillance: Conserving muskoxen and caribou in the Canadian Arctic. Biol. Conserv., 217, 337-348, doi: 10.1016/j.biocon.2017.11.010.
Tryland, M., and S. J. Kutz, 2018: Reindeer and Caribou: Health and Disease. CRC Press, 534pp.
November 28, 2018